Research Projects
GPCR signaling assemblies, neuromodulation and synaptic wiring in vision & reward circuits
The main emphasis of research in the Martemyanov laboratory is on understanding the fundamental principles of signal processing by G protein coupled receptor (GPCR) systems and their pharmacology and role in disease. GPCRs constitute the largest family of cell surface receptors in mammalian genomes, endowing cells with an ability to sense their environment and communicate with each other. Their evolutionary success is underscored by a wide spectrum of extracellular stimuli that they detect, including light, ions, hormones, and neurotransmitters, and their versatile signaling to a vast number of intracellular targets, such as enzymes, ion channels and transcription factors. Accordingly, dysfunctions in GPCR systems are linked to many diseases. Furthermore, altering the activity of GPCRs pharmacologically often produces clinically beneficial outcomes regardless of the disease origin, and GPCRs are targeted by ~50% of current drugs.
Our research program is based on three pillars. First, understanding intricate mechanistic details governing operation of GPCR signaling assemblies that are increasingly viewed as “signalosomes” and understanding its role in neuronal physiology and animal behavior using mouse genetics. Second, uncovering ‘dark’ biology of missing regulatory elements in GPCR pathways by unbiased genomic and proteomic approaches. Third, devising and applying novel optical tools for interrogation of signaling versatility of GPCRs in the endogenous setting of the nervous system. Currently, our laboratory pursues the following directions.
Role of GPCR signalosomes in trans-synaptic signaling and neuronal wiring
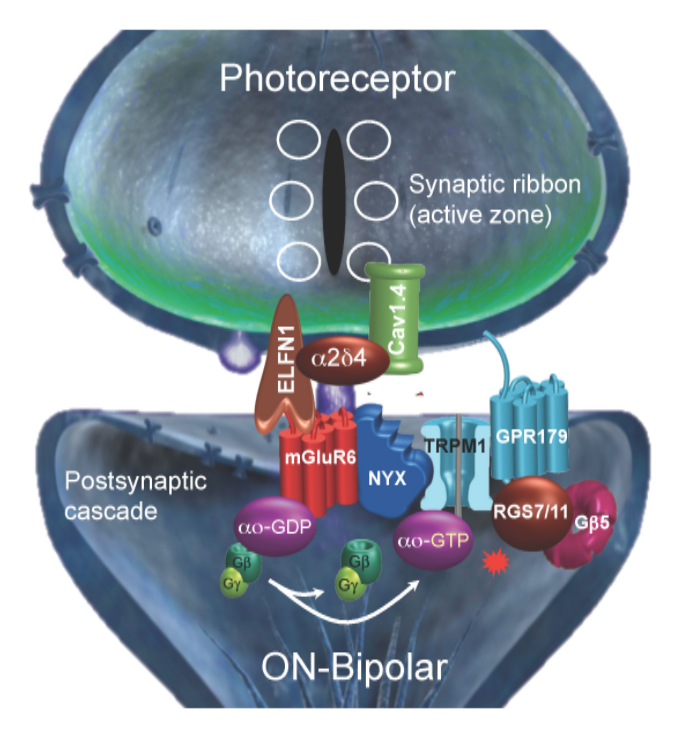
In our studies of photoreceptor synapses, we have recently made a series of observations that we believe reflect a general organizational principle of many GPCR systems in the nervous system. Synaptic communication of rod and cone photoreceptors with downstream ON bipolar neurons (ON-BC) is a critical point in the relay of a light stimulus to the brain. Here, light-induced changes in the release of neurotransmitter glutamate by photoreceptors are sensed by a GPCR, mGluR6, on postsynaptic ON-BCs.
In addition to mediating synaptic communication with photoreceptors, mGluR6 is also essential for the physical synapse formation, a process that occurs with remarkable selectivity, such that ON-BCs discriminate between rods or cones. These features make photoreceptor synapses a convenient model for addressing many fundamental questions pertaining to spatio-temporal aspects of GPCR signaling and synaptic selectivity. Over the last several years, my laboratory substantially contributed to deciphering the molecular organization of this GPCR cascade while developing the ‘signalosome’ model. In ON-BC neurons, mGluR6 is scaffolded with the effector channel TRPM1 and the Regulator of G protein Signaling (RGS) complex.
These interactions are mediated by the leucine-rich repeat (LRR) protein NYX and orphan receptor GPR179. In addition, the complex is anchored via extracellular interactions involving direct trans-synaptic binding of mGluR6 with a pre-synaptic LRR protein ELFN1, which in turn is scaffolded together with the CaV1.4/a2d4 channel complex controlling glutamate release. We are working to define the full composition and structure functional organization of the trans-synaptic complex using a variety of approaches including electrophysiology, structural biology and biochemistry. We are also investigating cell biological mechanisms of photoreceptor wiring by defining synaptic selectivity code of photoreceptors by studying the role of newly identified cell adhesion molecules.
Terra incognita of G protein regulatory networks in the striatum
Ultimately, we are interested in understanding the complexity of GPCR signaling systems in the endogenous setting where they often work in parallel integrating multiple concurrent signals. One of the best models for studying GPCR signaling is provided by the medium spiny neurons (MSN) in the striatum, a structure involved in processing reward and movement-related decisions. Striatal MSNs process a staggering number of neurotransmitters inputs for which they utilize a number of GPCRs. However, they can discriminate between individual GPCR-mediated signals while adjusting sensitivity based on experience and demands with incredible plasticity to drive unique behaviors.
We have been particularly fascinated by the opioid system that exerts powerful and lasting effects on reward valuation. Targeted by the most effective and widely used painkillers, it is a clinical marvel and at the same time a societal liability with millions of people succumbing to addiction. We use the opioid system as a behavioral model for interrogating GPCR signaling mechanisms in striatal neurons.
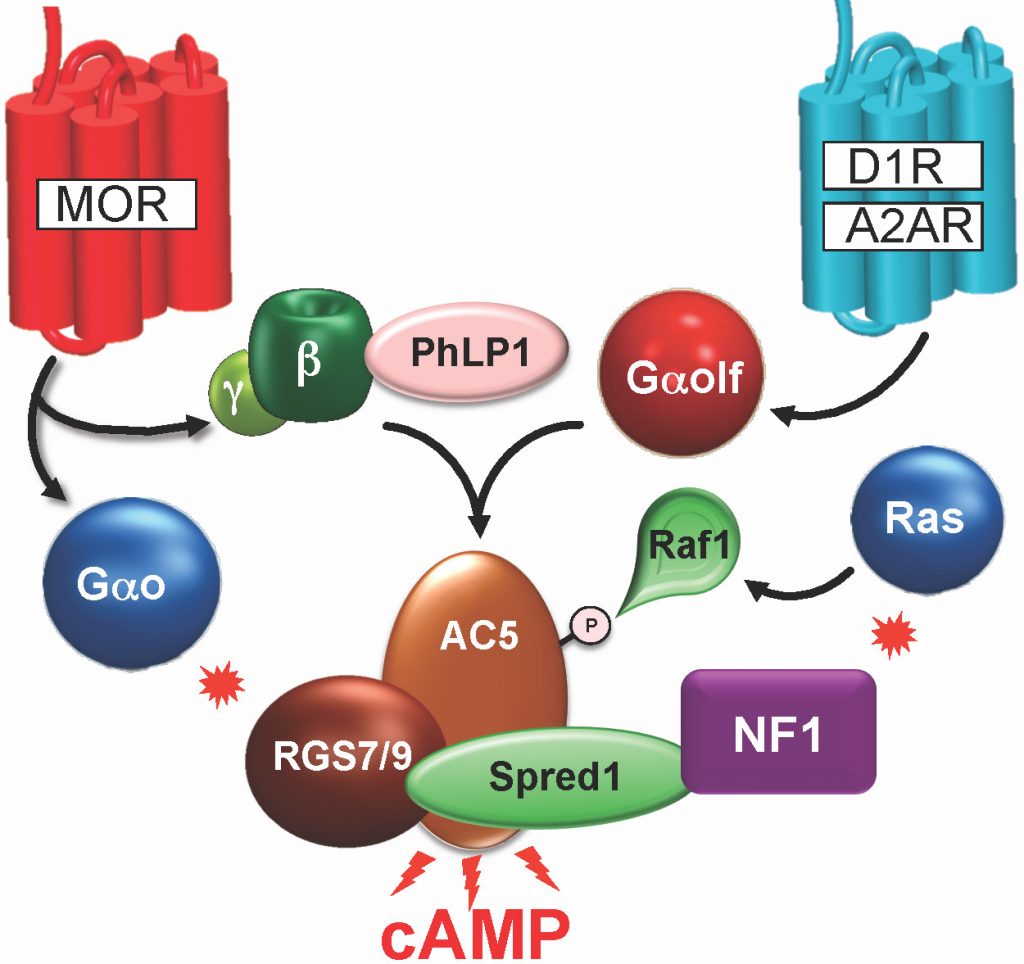
In this program we are defining new elements of the GPCR signal processing complex. Over the years of conducting proteomic and genetic screens and following them up with functional studies in mice, we identified several such novel components and mechanisms which we believe to be of general relevance for processing GPCR signals. For example, sensitivity of signaling is controlled by a complex of RGS proteins involving at least 4 subunits (RGS7, RGS9-2, R7BP, Gb5) scaffolded in a signaling complex with AC5, the main effector enzyme producing cAMP and its activator Gaolf with the help of a chaperone-like assembly factor PhLP1. More recently, we found an alternative branch of signaling that involves direct activation of Ras regulator NF1 by G proteins, tapping into regulation of kinase pathways to produce long lasting effects and boosting opioid efficacy. Our research is aimed at characterizing the logic of connecting individual elements in the complex, their interplay and the functional consequences of disrupting interactions in the complex. Much of these studies will be looking at the impact on generating a cAMP signal in parallel with analyzing behavioral responses of mice to addictive opioids.
Illuminating unconventional biology of orphan receptors
Much of the research on GPCRs is focused on neurotransmitter receptors with conventional signaling properties. However, a significant fraction of GPCRs, the so-called ‘orphan’ receptors, remain poorly explored likely due to their unorthodox biology. While profiling gene and protein expression we found GPR158 to be one of the most abundant orphan GPCRs in the brain. It shows prominent regulation by maladaptive states such as exposure to stress and drugs. Notably, we found that GPR158 does not signal via heterotrimeric G proteins and instead links to an RGS complex, allosterically regulating its activity.
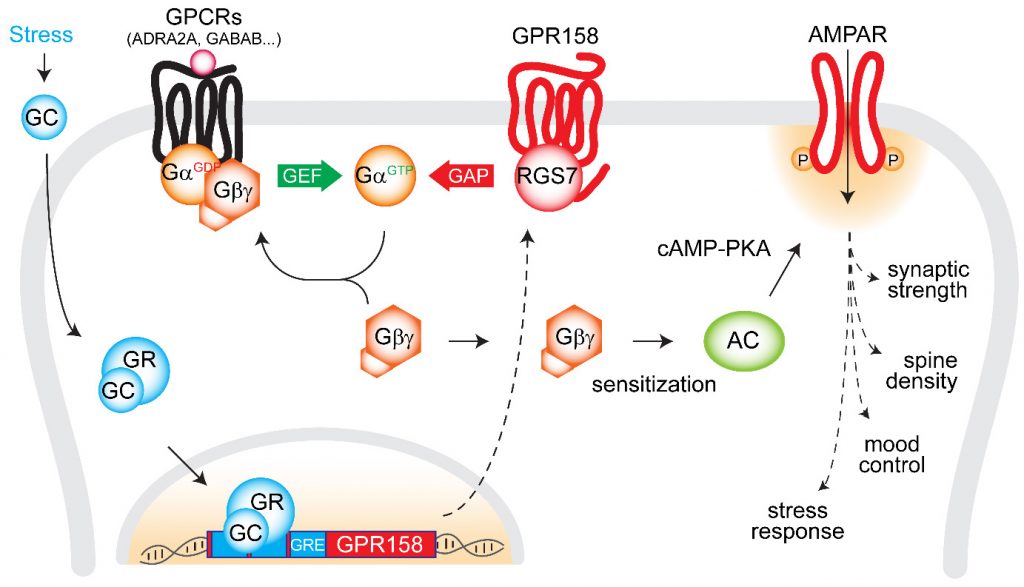
Conceptually, this introduces a novel paradigm that orphan GPCRs can produce cellular response not only by activating G proteins but also by sending the opposite signal deactivating them. We are actively pursuing the biology of GPR158 and the related receptor, GPR179, studying their role in shaping signaling cascades as well as behavioral implications. Further studies also focus on other poorly understood members of GPCR family including adhesion GPCRs and putative peptide receptors in synaptic physiology, circuit formation and behavior.
Building tools and approaches for deciphering GPCR signaling logic
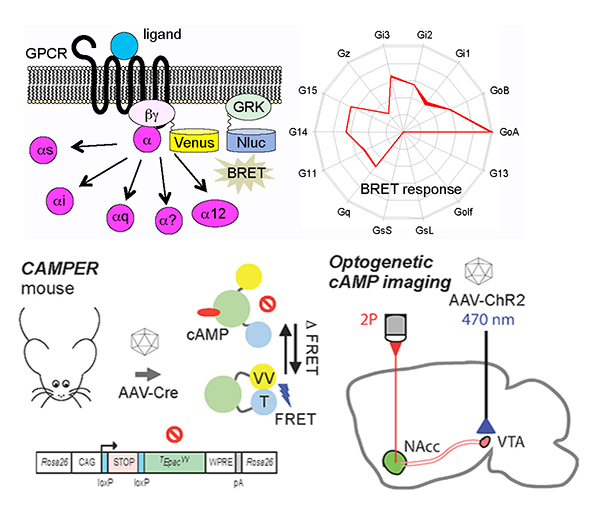
The laboratory is embarking on the challenge of understanding the complexity of GPCR-mediated signal conversion process in vivo. We have recently developed a “molecular dactyloscopy’ platform for interrogating complexity of GPCR coupling to their G protein substrates, studying their pharmacological biases and relevance to neuropsychiatric disease affecting GPCR signaling systems.
In this approach we measure the ability of GPCRs to activate their multiple G protein substrates in living cells using BRET-based real-time quantitative readout. We have found that GPCRs exhibit unique profiles of interactions with a gamut of Ga subunits, creating a characteristic “fingerprint”, which we think cells utilize for discriminating between individual commands encoded by messages.
We are currently building comprehensive G protein specificity maps for all GPCRs, interrogating pharmacological biases of FDA approved GPCR drugs and studying pharmacogenomics aspects of GPCR variability in normal human population and pathological conditions. We have also recently developed an in vivo tool that allows monitoring signaling by many GPCRs by following their downstream effects on cAMP dynamics We are using this model to determine the integration of multiple concurrent inputs in intact neural circuits by imaging cAMP dynamics in response to optogenetically activating projections that release different neuromodulators (glutamate, GABA, acetylcholine), while blocking individual receptors pharmacologically and genetically.
Discovery of small molecule probes for RGS complexes
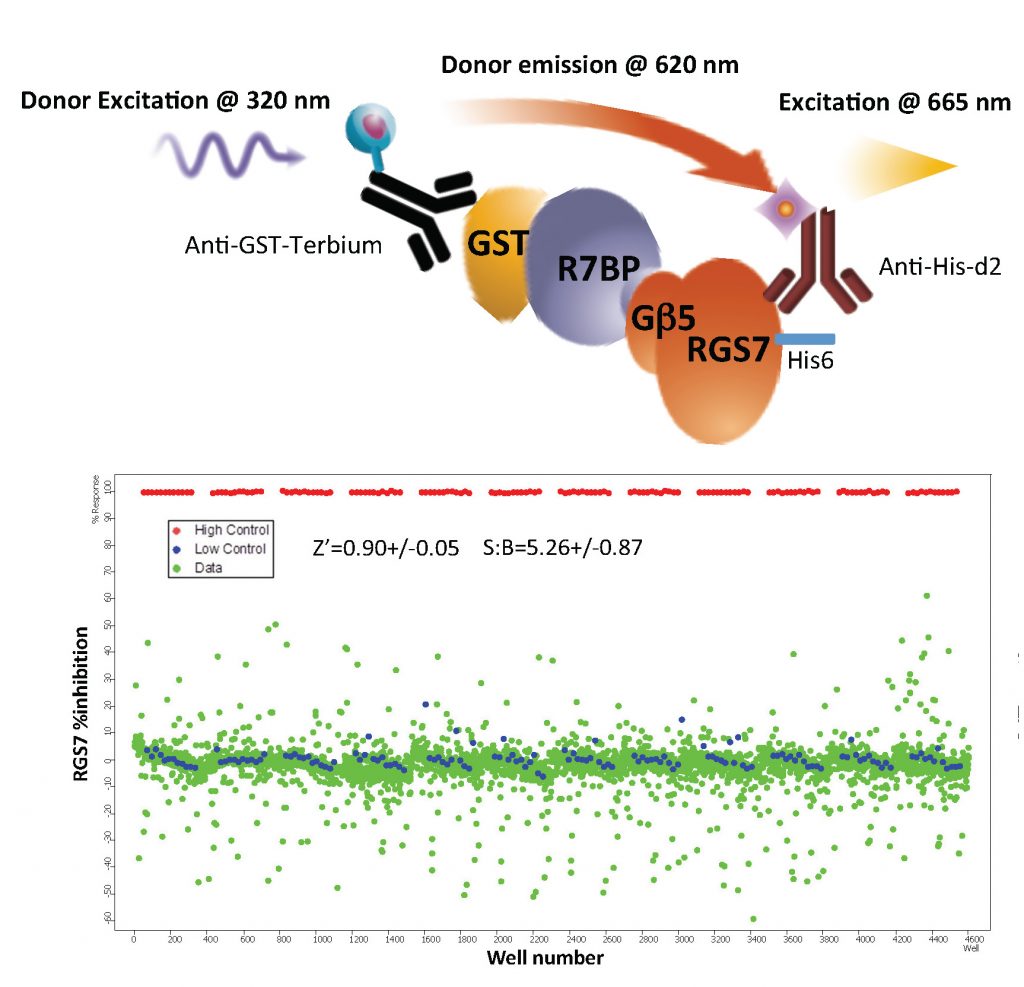
Regulators of G protein signaling are emerging as attractive targets for drug development. As a negative regulators of GPCR signaling they hold promise for designing intervention strategies aimed at increasing precision and efficacy of GPCR medications. In our research on modulation of opioid signaling in the striatum we discovered key role of RGS7-Gb5-R7BP complex in controlling behavioral sensitivity of opioid analgesics acting on mu-opioid receptor. In particular genetic suppression of this RGS complex changed the effects of opioids augmenting their analgesic effects while reducing dependence and tolerance. We are currently developing small molecule inhibitors of RGS7 complex with the hope of improving the safety profile of opioid drugs. To achieve this purpose we use a series of innovative HTS assays to discover and characterize useful leads.